The vast majority of oil refineries are equipped with a catalytic reforming unit that fulfils three main functions: production of high-octane oil cuts for gasoline production (known as reformates), production of aromatic-rich cuts containing fewer than 10 carbon atoms, used in the chemicals industry, and generation of dihydrogen, primarily used in hydrotreatment and hydrocracking units.
For naphthaa reforming units , a close but non-rationalized link connects the distribution of products exiting the process, and hence selectivity, to the formulation of the active catalyst phase. One of the solutions under consideration to provide a better understanding of this link involves developing kinetic models capable of integrating some of the catalyst’s physicochemical properties (catalytic descriptors) as input parameters.
The integration of these catalytic descriptors in kinetic models would then make it possible to better anticipate the effect of active phase changes on the catalytic performance of processes but also to propose innovative formulations to maximize catalyst selectivity.
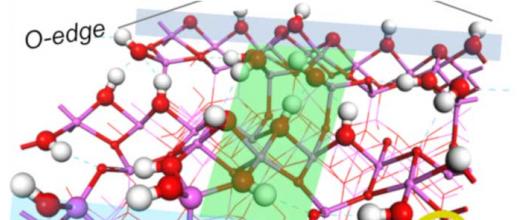
For this purpose, a PhD projectb focused on describing the links between a catalyst’s selectivity and its formulation. This project studied the bi-functionality of the catalyst (Pt/Al2O3-Cl) used for the naphtha reforming reaction. This case is complex since certain reactions only take place on acid sites created by the presence of Cl, others on metal - Pt - sites and others still require the presence of both sites, with, in this case, the influence of distance on catalyst selectivity. The challenge with this research lay in the identification of easily quantifiable, controllable and adjustable selectivity descriptors.
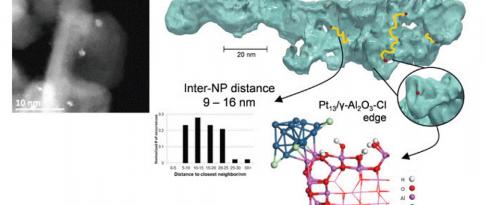
A kinetic study of this reaction for n-heptane was conducted for 24 catalysts, covering various platinum and chlorine concentrations, and using different supports, in order to vary the descriptors of both the acid (Cl content, Pt-Cl distance) and metal (Pt content) phases. This experimental study was conducted for each catalyst in an HTE unitc. The kinetic constants for each catalyst were first estimated via a power law model and then correlated with the descriptors.
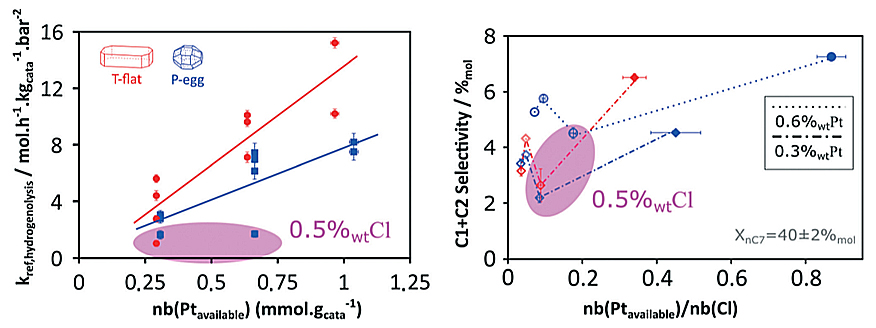
The results obtained shed light on the catalyst formulation/selectivity relationship and reveal hitherto unexpected phenomena concerning the effect of chlorine on the metal activity of platinum particles [1,2]. In particular, surprising results were obtained concerning hydrogenolysis reactivityd (figure):
- a preferential activity on one of the catalyst supports, with a minimum of activity at intermediate chlorine concentrations;
- a link between activity and chlorine concentration.
By combining the modeling approach with high-throughput experimentation and the use of sophisticated characterization methods (proton NMR, H2/O2 assays, HR-TEM, ab initio calculations and XAS), this research resulted in significant progress towards the development of kinetic models for reforming. These models integrate catalytic descriptors as input data. The research also highlighted unexpected behaviors that will be exploited as potential avenues for innovation in the development of new catalysts.
The task now is to extrapolate the findings to actual reforming feeds and optimize characterization of the active catalyst phase, via a model reaction adapted to mild acidic supports.
a- Liquid light hydrocarbon mixture obtained from crude oil distillation
b- Thesis title: “Identification of active phase catalytic descriptors in the reforming of n-heptane”
c- High-throughput experimentation
d- Chemical reaction whereby a covalent carbon-carbon bond or carbon-heteroatom single bond is cleaved by hydrogen
References:
- O. Said‐Aizpuru, A.T.F. Batista, C. Bouchy, V. Petrazzuoli, F. Allain, F. Diehl, D. Farrusseng, F. Morfin, J.‐F. Joly, A. Dandeu, Non Monotonous Product Distribution Dependence on Pt/γ‐Al2O3-Cl Catalysts Formulation in n‐Heptane Reforming, ChemCatChem 2020, 12. hal-02544492.
>> https://doi.org/10.1002/cctc.201902260
- O. Said‐Aizpuru, F. Allain, A. Dandeu , F. Diehl, D. Farrusseng, J.‐F. Joly, Kinetic modelling of Pt/γ-Al2O3-Cl catalysts formulation changes in n-heptane reforming, Reaction Chemistry & Engineering 2021,6. hal-03249638.
>> https://doi.org/10.1039/D1RE00073J
Scientific contacts: aurelie.dandeu@ifpen.fr ; florent.allain@ifpen.fr ; fabrice.diehl@ifpen.fr ; Jean-François Joly
You may also be interested in
Spectroscopy and quantum calculations uncover the secrets of alumina supports
Metal nanoparticles living on the edge
Platinum nanoparticles supported on chlorinated γ-alumina are used in bifunctional heterogeneous catalysts, which are central to numerous industrial processes. An atomic-scale study...