25.11.2020
5 minutes of reading
The quantities of soot resulting from the combustion of a raw material partly depend on particle formation conditions. Research conducted at IFPEN has established a reaction mechanism to describe a key step: the dimerization reaction. A new methodology now makes it possible to better predict the genesis of soot particles, helping to limit the associated health and environmental effects.
Soot, harmful to health and environment
Soot consistsessentially of carbon particles formed as a result of the incomplete combustion of raw material, for example, in IC engines, furnaces or forest fires. These soot (nano)particles are harmful to human health, because once inhaled they are deposited in the airways, causing irritation and damage. They can also adsorb other pollutants on their surface, especially volatile organic compounds that are equally harmful.
In this context, the smallest particles are the most harmful ones, since they penetrate the respiratory system more actively. Another worrying consequence of these soot particles in the atmosphere is their negative influence on global warming with a not insignificant contribution to radiative forcing.
Better understanding soot formation
The amount of soot particles generated during combustion and their (atomistic) nanostructure significantly depend on their formation conditions. Moreover, it is known that this nanostructure has a direct impact on soot reactivity, particularly with respect to oxygen (combustion). It is therefore important to have a better understanding of what exactly happens during the early stages of soot particle formation. One of the difficulties involved in accessing this knowledge stems from the fact that the information required relates to broad scales of time and space.
It is for this reason that a PhD thesis project1 conducted at IFPEN focused on this specific topic [1], via a multi-scale approach combining macroscopic kinetic simulations of gas-phase combustion (representative of a combustion chamber) with atomistic calculations [2]. By varying the temperature (from 1,200 to 2,000 K), the composition of the hydrocarbon and the hydrocarbon/oxygen ratio, combustion calculations generated a total of 144 polycyclic aromatic hydrocarbon (PAH) mixtures, offering a very broad range of molecular configurations (Figure 1).
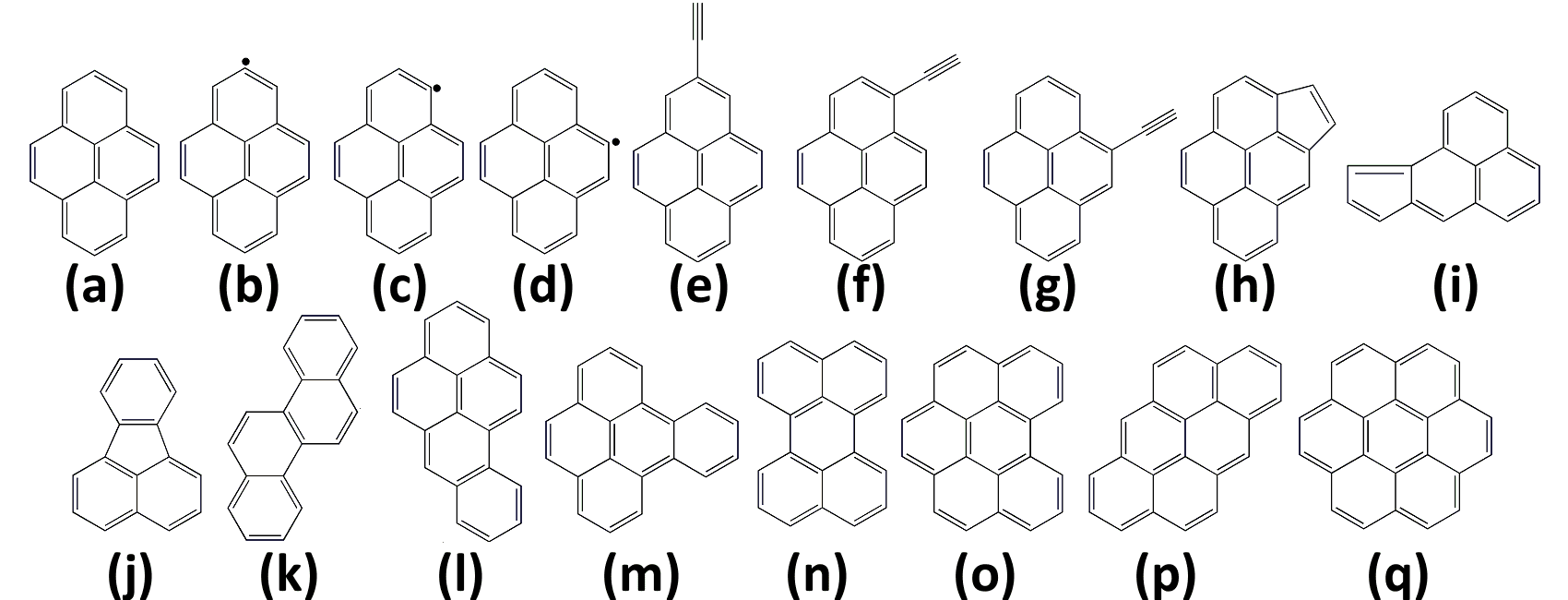
A reaction studied in detail: dimerization
On the basis of these calculated mixtures, molecular dynamic simulations were performed on the dimerization2 reaction between two PAH molecules, considered to be a key step in the formation of soot precursors.
These simulations, first of all, demonstrated that an increase in temperature promoted the dimerization reaction. It was then possible to categorize the resulting dimers into three broad families (Figure 2): directly-linked aromatics (A), pericondensed PAHs (B) and aromatics linked by an alkyl chain (C).
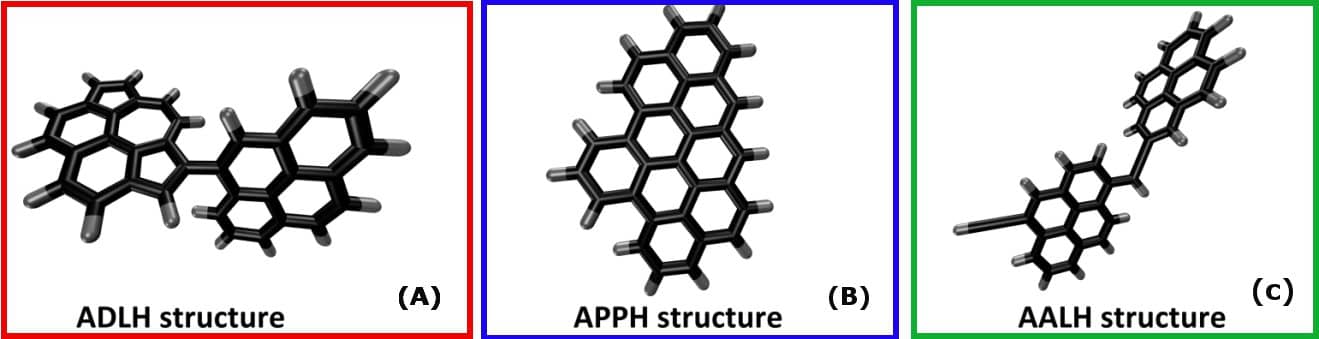
Thanks to the performed reactive molecular dynamics simulations, a general reaction mechanism could be established, describing the formation of the members of the three families and their interconversion (Figure 3).
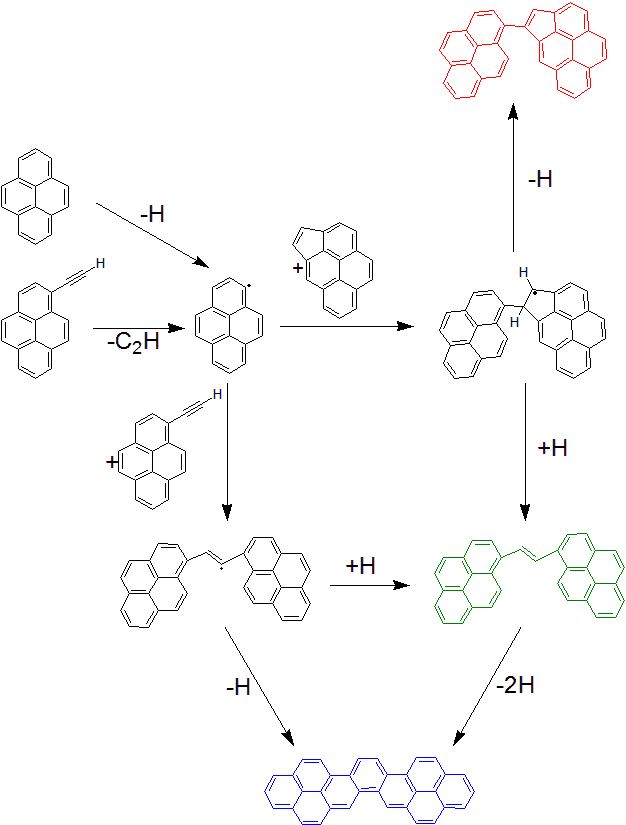
Atomic-scale simulations also showed that mixtures rich in aliphatic compounds3 foster the formation of structures in the (C) family and/or dimers including lateral aliphatic groups.
For a new prediction methodology
These results are now being used to support numerical combustion codes with a view to better predicting soot precursor formation as a function of operating conditions, at the same time incorporating the impact of the chemical composition of the fuel.
Thanks to this research, a new methodology now exists to anticipate and act on the genesis of soot particles in controlled combustion processes, thereby helping to limit the health and environmental effects of the equipment concerned.
1 Conducted within the framework of the IPPAD fundamental research project, as part of the ITN-MSCA Horizon 2020 program.
2 Chemical reaction whereby two (almost) identical molecules are combined.
3 Acyclic hydrocarbon.
Scientific contact : Theo de Bruin
References
[1] Development of a multi-scale approach using chemical kinetics and reactive force field molecular dynamics to model soot formation and oxidation, M. Keller, thèse doctorale, Institut Polytechnique de Paris, 2019.
[2] A Theoretical Multiscale Approach to Study the Initial Steps Involved in the Chemical Reactivity of Soot Precursors, M. Keller, T. de Bruin, M. Matrat, A. Nicolle, L. Catoire, Energy Fuels, 2019, 33 10255–10266. https://pubs.acs.org/doi/abs/10.1021/acs.energyfuels.9b02284





