22.09.2023
3 minutes of reading
Dihydrogen H2 has a bright future ahead in the drive for energy transition. However, it is seldom found in its natural state on Earth. Water electrolysis can produce carbon-free dihydrogen, provided that the electricity used is carbon-free. As part of the MoSHy project, which brings together three research laboratories including IFPEN, several methodologies are being developed, which combine experimentation and molecular modeling, in order to identify high-performance electrocatalysts that are also frugal in terms of scarce resources. A promising avenue that has captured the attention of researchers is the use of molybdenum sulfide (MoS2)-based active phases in PEM (Proton Exchange Membrane) electrocatalysts, which also opens up a possible conversion solution for these major ingredients in refining catalysts.
The quest for carbon-free hydrogen
Dihydrogen H2 is an essential molecule for major applications, such as fertilizer production, and it has also become essential for the energy transition, particularly for heavy mobility or even transient energy storage. This molecule, which is virtually non-existent in nature, has to be produced from natural resources. Water electrolysis is the only process capable of producing totally carbon-free dihydrogen, provided that the electricity used is also carbon-free. PEM technology (Figure 1) is currently one of the most effective electrolyzer technologies available to adapt to the intermittent production of electricity by certain rapidly-growing renewable energies, such as solar and wind power.
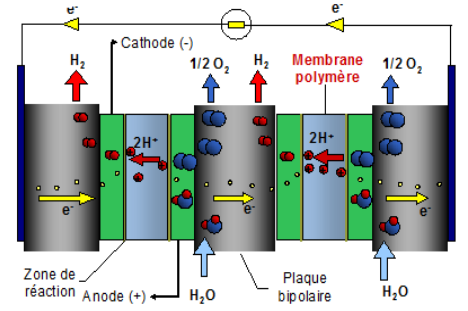
1 Excerpt from sheet 3.2.1 published by the AFHYPAC
Unfortunately, PEM uses large quantities of platinum, which is both expensive and scarce. For this reason, if Europe hopes to reach its target of deploying 80 GW of electrolyzers by 2030, it is essential to speed up the development of electrocatalysts2 that are as efficient as platinum-based ones, but for which the resource is not limited and therefore remains cost-effective.
2 Catalysts of electrochemical reactions
A new future for MoS2 phases?
A few years ago, molybdenum sulfide (MoS2)-based active phases, the basic structures of catalysts for hydrotreatment3 , emerged as interesting candidates to replace platinum in electrocatalysts. For this reason, in 2018, a consortium was set up to assess their potential through the MoSHy (MoS2 for Hydrogen) project, funded by the Auvergne Rhône-Alpes region as part of the “Pack Ambition Recherche” (Research Ambition Pack in English) In addition to IFPEN, it includes the chemistry laboratory of the Ecole Normale Supérieure de Lyon, a key player in atomistic modeling, and the LEPMI (Laboratory of Electrochemistry and Physical-Chemistry of Materials and Interfaces) in Grenoble, a laboratory specializing in electrochemistry. By combining experimental work and molecular modeling, this project aimed to develop a cost-effective and efficient electrocatalyst for hydrogen production.
3 Hydrocarbon treatment process using dihydrogen to remove certain elements present in the light fractions of petroleum (sulfur, nitrogen, poisons for catalysts)
Is molecular modeling a predictive tool?
MoS2 phase exhibits promising electrochemical performance, but the literature states that the basal plane of their crystal structure is inactive [1]. A possible improvement strategy would be to increase the number of active sites, and to evaluate the impact of substitution doping4 (figure 2). The ENS first identified 17 abundant elements to partially replace molybdenum and 5 elements to partially replace sulfur. For screening purposes, the team focused on adsorption competitions between the various reagents, as well as on two phenomena likely to hinder hydrogen production at these sites, namely the release of hydrogen sulfide (H2S) and the tendency for dopant segregation/dispersion.
4 Consists of incorporating atoms of another element into the crystal lattice of the initial material to replace certain initial atoms
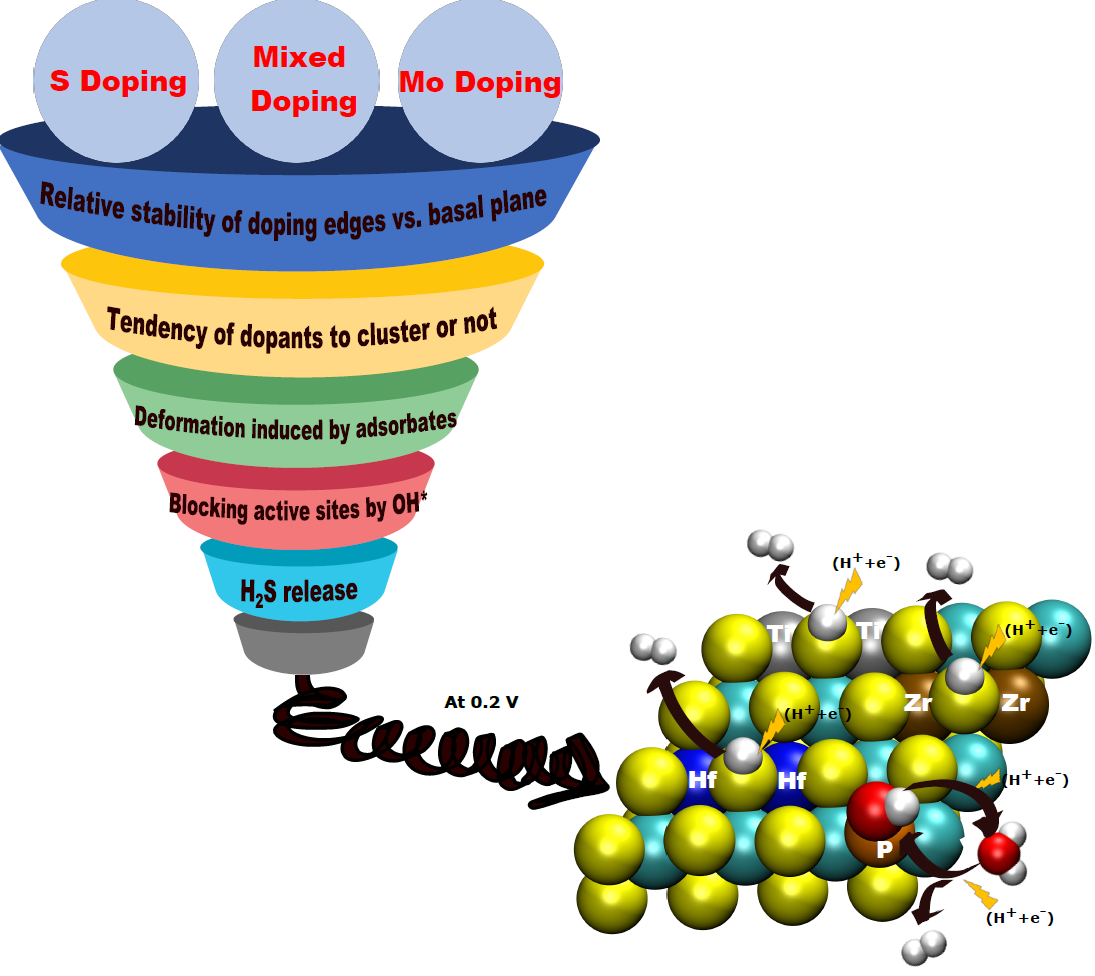
The results show that most of the dopants screened could significantly improve hydrogen production [2]. However, most of them are unstable in one way or another, and promising systems are few and far between: those doped with elements from group IV of the periodic table (Ti, Zr, Hf) or those containing phosphorus P instead of sulfur. Following the experimental results obtained in electrolysis, the most effective dopants from this point of view have now been patented.
Improvements in the formulation of MoS2 active phases are still being studied with the aim of replacing platinum in PEM electrolysers, and this experimental research will continue as part of a new thesis starting in autumn 2023.
5 Exploration of nano-architectures of MoS2 active sites for the hydrogen evolution reaction
Reference :
[1] How to dope the basal plane of 2H-MoS2 to boost the hydrogen evolution reaction? N. Abidi, A. Bonduelle-Skrzypczak, S. Steinmann, Electrochimica Acta 2023, 439, 141653. DOI:https://doi.org/10.26434/chemrxiv-2022-hqgjv
Scientific contact : Audrey BONDUELLE








