08.11.2023
3 minutes of reading
Solvents play an integral role in many industrial processes. Their thermodynamic properties are studied by IFPEN’s experts within the framework of collaborative projects and a dedicated chair at IFP-School. To reinforce scientific exchange, Fufang Yang, a young researcher based at the DTU, joined IFPEN’s teams to investigate several fundamental questions concerning the thermodynamics of solvents.
Within the framework of the European ElectroThermo1 project, led by the Technical University of Denmark (DTU), IFPEN’s Thermodynamics and Molecular Modeling Department hosted Fufang Yang, a visiting post-doctoral researcher from the DTU recently appointed to the post of young editor of Journal of Chemical & Engineering Data. The project’s theme was fully in line with our activities concerning electrolyte thermodynamics and also fed into the IFP-School chair, as well as the JIP of the same name, promoted by IFPEN.
A post-doctoral research residency dedicated to the study of complex mixtures
During his stay, the young researcher used a theoretical equation of state to study the activities of species, as well as their volatilities for mixtures containing salts in a mixed solvent (water + alcohol). The aim of this relatively fundamental research was to better describe solvent volatilities in biomass-based mixtures on the one hand and, on the other, the activities of ionic species, which are important for different usages: choice of solvent for metal extraction, study of corrosion by these species, battery function, etc.
Data consistency study
As is usual for this type of study, the method is based on comparing experimental data with models. Hence, the first part of the research consisted in gathering data and evaluating their quality (internal and external coherence), starting with a study conducted within the framework of the ELeTher JIP [1]. For a given chemical system, examining internal consistency consists in comparing all the available data concerning properties (activity of ions and solvents as well as caloric properties) and their compliance with the relevant laws of thermodynamics. Examining external consistency consists in identifying trends within the considerable quantity of data collected so as to extract information regarding molecular-level phenomena. The research conducted during this step for mixed water + alcohol solvents, with simple salts, led to two publications [2,3]. The first describes the use of a purely thermodynamic approach (see figure 1), while the second focuses on the use of a neural network.
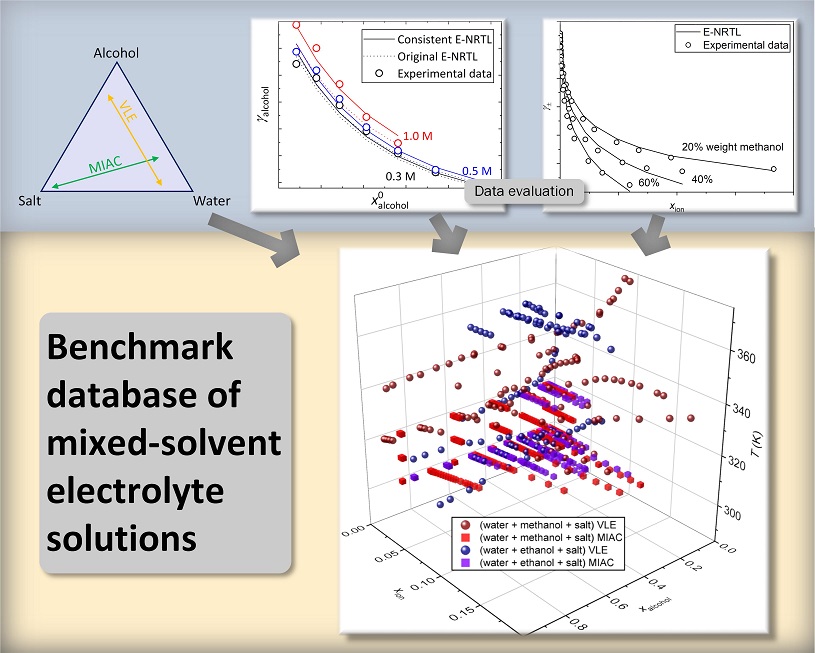
Does the predictive model actually reflect the data?
In the second phase of the research, the ePPC-SAFT model2 was used and parametrized with a view to describing the data as precisely as possible. This research phase, which built on a thesis completed at IFPEN3, highlights the interest of explicitly describing the interactions of ion pair formation and of ion-solvent interactions using a pseudo-chemical equilibrium. These results, already published for the aqueous solutions [4,5], and in the publication phase for the mixed solvents [6], will now be exploited within the framework of new doctoral research, in order to better understand the impact of different intermolecular interactions on solvent volatilities (see, for example, figure 2 showing how each energy contribution influences the ion activity coefficient).
Ultimately, this research will help shed light on the industrial needs addressed in the EleTher JIP and make it possible to construct a more efficient predictive model, insofar as it would require fewer input data.
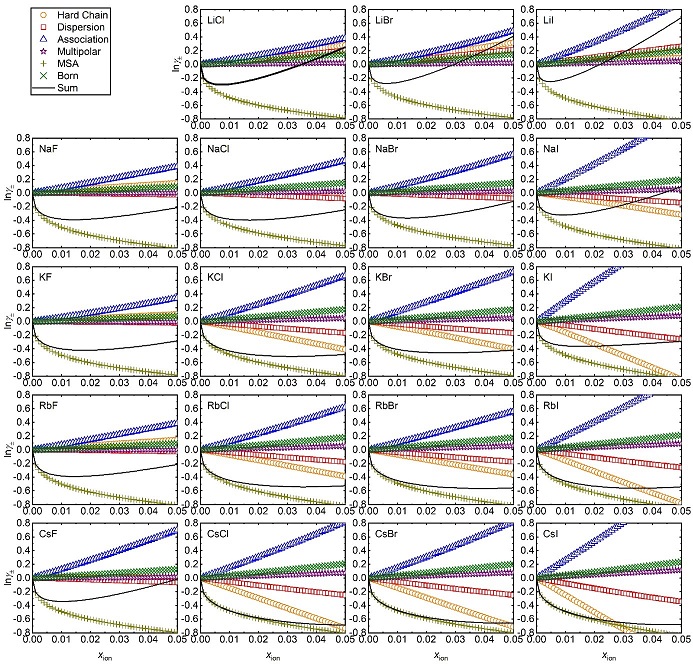
1 New paradigm in the field of electrolyte thermodynamics, ERC advanced grant project.
2 Electrolyte Polar Perturbed Chain Statistical Associating Fluid Theory.
3 Thesis by S. Roa Pinto “Modèle thermodynamique prédictif pour les systèmes complexes électrolytiques” (Predictive thermodynamic models for complex electrolytic systems).
References:
[1] S. Vaque Aura, J.-S. Roa Pinto, N. Ferrando, J.-C. de Hemptinne, A. ten Kate, S. Kuitunen, N. Diamantonis, T. Gerlach, M. Heilig, G. Becker, M. Brehelin, Data Analysis for Electrolyte Systems : A Method Illustrated on Alkali Halides in Water, J. Chem. Eng. Data 66 (2021) 2976–2990.
>> DOI: https://ifp.hal.science/hal-03298689
[2] F. Yang, T.D. Ngo, G.M. Kontogeorgis, J.-C. de Hemptinne, A Benchmark Database for Mixed-Solvent Electrolyte Solutions: Consistency Analysis Using E-NRTL, Ind. Eng. Chem. Res. 61 (2022) 15576–15593.
>> DOI: https://doi.org/10.1021/acs.iecr.2c00059
[3] F. Yang, J. Qu, G.M. Kontogeorgis, J.-C. de Hemptinne, Reference Density Database for 20 Aqueous Alkali Halide Solutions, J PHYS CHEM REF DATA 51 (2022) 43104.
>> DOI: https://doi.org/10.1063/5.0124173
[4] F. Yang, T.D. Ngo, J.S. Roa Pinto, G.M. Kontogeorgis, J.-C. de Hemptinne, Systematic evaluation of parameterization approaches for the ePPC-SAFT model for aqueous alkali halide solutions, Fluid Phase Equilib. 570 (2023) 113778. DOI: https://doi.org/10.1016/j.fluid.2023.113778
[5] F. Yang, G.M. Kontogeorgis, J.-C. de Hemptinne, Systematic evaluation of parameterization approaches for the ePPC-SAFT model for aqueous alkali halide solutions. II. Alkali bromides, iodides, fluorides, and lithium halides, Fluid Phase Equilib. 573 (2023) 113853.
>> DOI: https://doi.org/10.1016/j.fluid.2023.113853
[6] F. Yang, G. Kontogeorgis, de Hemptinne, Jean Charles, Composition-dependence of relative static permittivity in ePPC-SAFT for mixed-solvent alkali halides, Fluid Phase Equilib.
>> DOI: https://doi.org/10.1016/j.fluid.2024.114103
Scientific contact: Jean-Charles de Hemptinne
You may also be interested in
Electrolyte thermodynamics at IFPEN
The field of electrolyte thermodynamics is a strategic one for IFPEN since it contributes to a number of its existing technological innovations, as well as those currently being developed...








