Unlike hydrocarbons of fossil origin, the molecules derived from biomass are polar, due to the heteroatoms they contain. This difference on a molecular scale induces a more complex macroscopic behavior that must be taken into account when designing the processes where such mixtures are encountered.
Moreover, while a significant variety of molecules is encountered in oil fluids, their diversity is still considerably larger in biomass. This makes it impossible to calibrate traditional thermodynamic models by conducting measurements on each molecule or each combination. It is therefore essential to have predictive tools, based on theoretical considerations, to calculate the properties of these mixtures from the molecular structures.
The objective of the “Thermodynamics for biofuels” teaching and research Chair was to construct such a predictive method.
To do so, researchers drew on academic research initiated in the 1990s, proposing methods based on statistical mechanics to construct thermodynamic models (SAFT equations of state).
A non-exhaustive list of research projects conducted within the framework of the Chair, particularly through PhD theses, illustrates the advances obtained:
- a method based on group contributions was used to calculate phase equilibria for mixtures of molecules identified in biomass[1]. The result is the GC-PPC-SAFT model, the name of which reflects its content[2,3];
- to compensate for the limits of the model for molecules with several oxygenated functionalities, a methodology was developed to extend group contributions, taking into account the distance between groups[4];
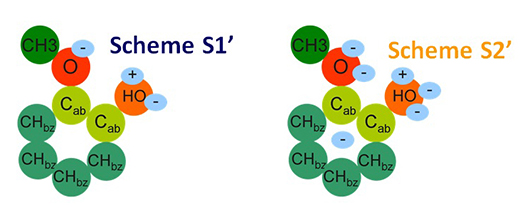
- for some biomass molecules with highly complex forms, such as guaiacol, the molecular description had to be adapted to the solvent containing the molecule (as illustrated in the diagram below)[5];
- the model developed was also compared to solvation measurements (interaction of a solute with a solvent) during joint research conducted with a team from Kazan University[6];
- GC-PPC-SAFT was also employed in the context of the esterification processes used to convert vegetable oils into fuel[7];
- finally, in order to dimension the hydrotreatment process for fluids derived from biomass, thereby making them compatible with oil fuels, the solubility of hydrogen in these fluids was studied[8].
Click on the picture to enlarge
Thanks to the work carried out within this research Chair, IFPEN has an “equation of state”-type tool available for better understanding and describing mixtures derived from biomass, with their oxygenated compounds. It is now widely used in applied research projects.
1- Tuck Foundation chair, 2009 to 2018
2- Statistical Associating Fluid Theory
3- Consisting in dividing molecules into fragments, less numerous than the molecules (example illustrated), with each considered to partly contribute to the desired property
4- GC for group contributions and P for the polar term, all based on the Perturbed Chain SAFT variant
[1] D. Nguyen-Huynh, J.-C. de Hemptinne, R. Lugo, J.-P. Passarello, P. Tobaly, Simultaneous liquid–liquid and vapour–liquid equilibria predictions of selected oxygenated aromatic molecules in mixtures with alkanes, alcohols, water, using the polar GC-PC-SAFT (Prédictions simultanées d'équilibres liquide-liquide et vapeur-liquide de certaines molécules aromatiques oxygénées dans des mélanges avec des alcanes, des alcools et de l'eau, à l'aide du GC-PC-SAFT polaire), 92 (2014) 2912–2935.
>> https://www.sciencedirect.com/science/article/pii/S0263876214002470
[2] T.-B. Nguyen, J.-C. de Hemptinne, B. Creton, G.-M. Kontogeorgis, Characterization Scheme for Property Prediction of Fluid Fractions Originating from Biomass (Schéma de caractérisation pour la prédiction des propriétés des fractions fluides issues de la biomasse), ENERG FUEL 29 (2015) 7230–7241.
>> http://dx.doi.org/10.1021/acs.energyfuels.5b00782
[3] T.-B. Nguyen, J.-C. de Hemptinne, B. Creton, G.-M. Kontogeorgis, Improving GC-PPC-SAFT equation of state for LLE of hydrocarbons and oxygenated compounds with water (Amélioration de l'équation d'état GC-PPC-SAFT pour la LLE des hydrocarbures et des composés oxygénés avec de l'eau), Fluid Phase Equilib. 372 (2014) 113–125.
>> http://www.sciencedirect.com/science/article/pii/S0378381214002064
[4] M. Jaber, W. Babe, E. Sauer, J. Gross, R. Lugo, J.-C. de Hemptinne, An improved group contribution method for PC-SAFT applied to branched alkanes: Data analysis and parameterization (Une méthode de contribution de groupe améliorée pour PC-SAFT appliquée aux alcanes ramifiés: analyse et paramétrage des données), Fluid Phase Equilib. 473 (2018) 183–191.
>> https://www.sciencedirect.com/science/article/pii/S0378381218302504
[5] L. Grandjean, J.-C. de Hemptinne, R. Lugo, Application of GC-PPC-SAFT EoS to ammonia and its mixtures (Application de GC-PPC-SAFT EoS à l'ammoniac et ses mélanges), Fluid Phase Equilib. 367 (2014) 159–172.
>> http://dx.doi.org/10.1016/j.fluid.2014.01.025
[6] M.-A. Varfolomeev, R.-N. Nagrimanov, M.-A. Stolov, N. Ferrando, R. Lugo, J.-C. de Hemptinne, Guaiacol and its mixtures: New data and predictive models. Part 2: Gibbs energy of solvation (Guaiacol et ses mélanges: nouvelles données et modèles prédictifs. Partie 2 : énergie de solvatation de Gibbs), Fluid Phase Equilib. 470 (2018) 91–100.
>> https://www.sciencedirect.com/science/article/pii/S0378381218301420
[7] C.-G. Pereira, L. Grandjean, S. Betoulle, N. Ferrando, C. Fejean, R. Lugo, J.-C.-de Hemptinne, P. Mougin, Phase equilibria of systems containing aromatic oxygenated compounds with CH4, CO2, H2, H2S, CO and NH3: Experimental data and predictions (Équilibres de phase des systèmes contenant des composés oxygénés aromatiques avec CH4, CO2, H2, H2S, CO et NH3 : Données expérimentales et prédictions), Fluid Phase Equilib. 382 (2014) 219–234.
>> http://www.sciencedirect.com/science/article/pii/S0378381214004567
[8] T.-K.-H. Trinh, J.-P. Passarello, J.-C. de Hemptinne, R. Lugo, Use of a non additive GC-PPC-SAFT equation of state to model hydrogen solubility in oxygenated organic compounds (Utilisation d'une équation d'état GC-PPC-SAFT non additive pour modéliser la solubilité de l'hydrogène dans des composés organiques oxygénés), Fluid Phase Equilib. 429 (2016) 177–195.
>> http://www.sciencedirect.com/science/article/pii/S0378381216303776
[9] T.-K.-H. Trinh, J.-C. de Hemptinne, R. Lugo, N. Ferrando, J.-P. Passarello, Hydrogen Solubility in Hydrocarbon and Oxygenated Organic Compounds (Solubilité de l'hydrogène dans les composés organiques hydrocarbonés et oxygénés), J. Chem. Eng. Data 61 (2016) 19–34.
>> https://pubs.acs.org/doi/full/10.1021/acs.jced.5b00119
Scientific contact: Jean-Charles De Hemptinne







