Thesis prepared by Edoardo Basilico: "Carbon steel corrosion scales protectiveness study in CO2 aqueous solution"
Due to their low cost and their good mechanical properties, carbon steels are a widely used material, including for equipments in contact with harsh environments, such as aqueous media containing CO2. In the latter, carbon steels are subject to uniform corrosion, resulting in some conditions to the formation of a pseudo-passive layera of iron carbonate on its surface, which protects it and stops the process.
However, sporadic contamination of the environment by oxygen can alter this protection and affect the reliability of installations exposed to this risk. The effect of some environmental parameters (composition of the water and the gaseous atmosphere) on the pseudo-passivation phenomenon thus represents a major challenge for the technological processes concernedb.
The influence of the composition of the corrosive environment was the focus of this thesis. The research was conducted within the framework of an experimental program in which parameters were varied around a reference condition leading to pseudo-passivation [1]. A combination of several experimental techniques was deployedc making it possible to show that the pseudo-passive layer was composed almost uniquely of siderite (FeCO3), with the possible presence of a magnetite precipitates (Fe3O4) at the inner interface between the corrosion scale and the steel.
The impact on the pseudo-passive layer of contamination of the environment by oxygen traces was evaluated by varying its concentration between 90 and 300 ppbd . The presence of oxygen modifies the mode of corrosion, shifting from a global and uniform attack to a localized corrosion, characterized by pits. Within these pits, phases other than siderite form, such as chukanovite (Fe2(OH)2CO3).
These results were then modeled using a mechanism [1, 3]: a first step is the formation by oxygen of cavities in the siderite layer formed prior to contamination (Figure 1). In these cavities, the oxygen is consumed and an oxygen depletion is produced, creating differential aeration conditions between the cavity and the rest of the deposit and the chukanovite formation.
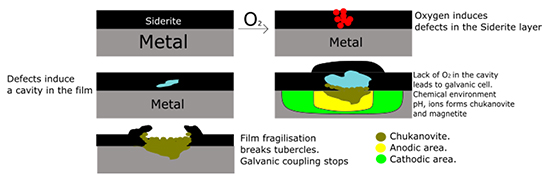
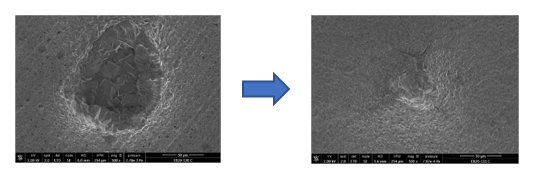
In addition to the mechanism involved, the results of this study highlighted the existence of an threshold oxygen concentration, below 90 ppb of oxygen, to initiate localized corrosion of the pseudo-passive surface. However, an important practical point is that when contamination stops, a pseudo-passive layer is reformed, restoring excellent corrosion resistance (Figure 2).
a- A layer with a thickness of a few microns, made up of corrosion products, which acts as a shield against the transport of iron ions into the solution.
b- For example: CO2 capture, transport and conversion, underground gas storage, conversion of biomass into fuels and chemicals.
c- Electrochemical impedance measurement, surface chemical analysis and local pH measurements.
d- i.e., contamination of 0.5 to 1.8% of oxygen in the gas.
Bibliographic references:
-
R.de Motte, E. Basilico, R. Mingant, J. Kittel, F. Ropital, P. Combrade, S. Neicib, V. Deydier, D. Crusset, S. Marcelin, "A study by electrochemical impedance spectroscopy and surface analysis of corrosion product layers formed during CO2 corrosion of low alloy steel", Corrosion Science, 2020, Volume 172 1 August Article 108666
>> https://doi.org/10.1016/j.corsci.2020.108666
-
E. Basilico, S. Marcelin, R. Mingant, J. Kittel, M. Fregonese, F. Ropital, "The effect of chemical species on the electrochemical reactions and corrosion product layer of carbon steel in CO2 aqueous environment: A review", Materials and Corrosion, 2021, 1, 16
>> https://doi.org/10.1002/maco.202012118
-
E. Basilico, S. Marcelin, R. Mingant, J. Kittel, M. Fregonese, J. Owens, R. Barker, A. Neville, F. Ropital, "Effect of O2 contamination on carbon steel pseudo-passive scales in CO2 aqueous solutions", Corrosion Science, 2022, Vol 205, Article 110388
>> https://doi.org/10.1016/j.corsci.2022.110388
Scientific contact: François Ropital
You may also be interested in
“From material to structure” modeling: the case of anchor cables for offshore wind, in corrosive environment
Anchor lines, the majority of which are carbon steel cables, are essential components for the stability of offshore floating structures, such as those supporting wind turbines. To overcome the risk of breakage during service, redundant lines are generally incorporated at the design stage, which adds significantly to the cost...