11.02.2020
5 minutes of reading
For the past few years, IFPEN has been developing a methodology aimed at gaining a better understanding of physicochemical phenomena and calibrating empirical thermodynamic laws using in silico calculations based on a molecular simulation approach. This methodology has just been applied to a new energies theme: geological hydrogen storage.
Hydrogen underground storage: a challenge for the energy transition
Hydrogen (H2) is set to play an increasingly important role in the energy mix in the coming years. Produced by electrolysis from renewable sources, it represents a very interesting energy vector to support the energy transition. In addition, its injection into the underground environment is proving to be an effective solution for its large-scale, long-term storage.
In this context, models are required to gain a clearer understanding of how hydrogen behaves in geological formations. In particular, its solubility in brines is a key parameter with a direct influence on its reactivity and mobility in porous media.
Molecular simulation to support traditional experiments
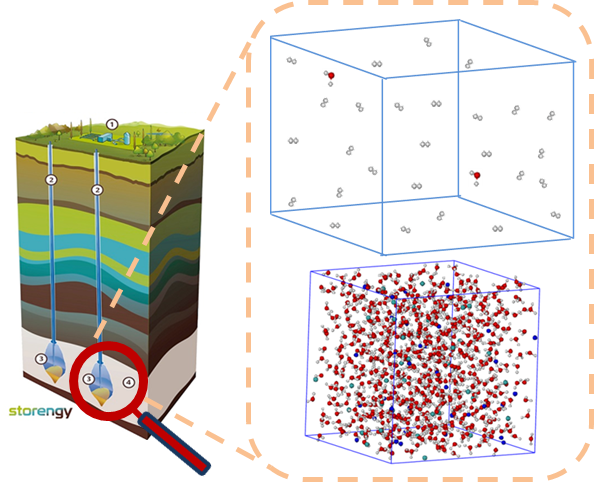
The lack of experimental phase equilibrium data relating to hydrogen and salt water in thermodynamic conditions representative of the underground environment significantly limits the development of such models. The very low solubility and potential dangers of this gas also make the acquisition of new data difficult and expensive. One alternative is therefore to supplement traditional experiments with molecular simulation calculations using the Monte Carlo method, which is particularly well suited to the prediction of phase equilibria.
Generating equilibrium data for calibration operations
Since it is based on rigorous physics, molecular simulation enables the precise prediction of the thermodynamic properties of interest. The methodology (see box) can be likened to in silico experimentation. First of all, a force field (set of equations describing the interactions between molecules) is parameterized and validated on the basis of existing experimental data. The predictive aspect of the method is then exploited to simulate the system in extrapolated temperature, pressure and salinity conditions typically encountered during storage operations. The in silico data thus generated can then be used to calibrate the thermodynamic laws used by operators.
This method, which gave rise to two publications, was implemented in partnership with Engie then Storengy: the molecular simulation results have been first validated for temperatures and salinities for which experimental data exist, and then simulations were extrapolated to higher levels more representative of geological storage sites. The results led to the precise calibration of an electrolyte equation of state (e-PPC-SAFT), which Storengy used to assess the impact of several hydrogen injection-extraction cycles on storage performance and the quality of the gas produced in a fictitious salt cavern.
Scientific contacts: Nicolas Ferrando, pierre.bachaud@ifpen.fr
References:
- Lopez-Lazaro, C.; Bachaud, P.; Moretti, I.; Ferrando, N. Predicting the phase behavior of hydrogen in NaCl brines by molecular simulation for geological applications. BSGF-Earth Sciences Bulletin 2019, 190, 7.
- Roa Pinto, J. C.; Bachaud, P.; Fargetton, T.; Ferrando, N.; Jeannin, L.; Louvet F. Modeling phase equilibrium of hydrogen and natural gas in salt caverns. In preparation.





