Thesis by Yacine Boudjema: « Effet du catalyseur zéolithique et de la nature du substrat sur les mécanismes de transformations des sucres » (Effect of the zeolitic catalyst and the nature of the substrate on the mechanisms of sugar transformation).
Biomass conversion using chemical products and intermediates is increasingly being adopted to reduce the carbon footprint of this industry. Among biomass-based resources, sugars are extremely attractive since they contain a lot of functional groups enabling their conversion into products of interest (alcohols, acids, etc.). This conversion requires the use of catalysts, including heterogeneous catalysts based on zeolites with Lewis acidity1, which have demonstrated strong potential, due to the presence of a tetravalent metal in the lattice position (Figure 1). This is the case with M-Beta zeolites, which can catalyze certain sugar conversions (Figure 2) while offering good recyclability. While at temperatures below 100°C these zeolites are capable of efficiently catalyzing important sugar interconversion reactions2, at higher temperatures (120-150°C) these zeolites also catalyze retro-aldol reactions, key C-C bond cleavage reactions for the formation of short-chain C2 (such as ethylene glycol) or C3 (such as lactic acid) chemical products. In addition, depending on the sugar undergoing the retro-aldol reaction, C2 products (from glucose), C3 products (from fructose), or a mixture of both (from xylose) are obtained.
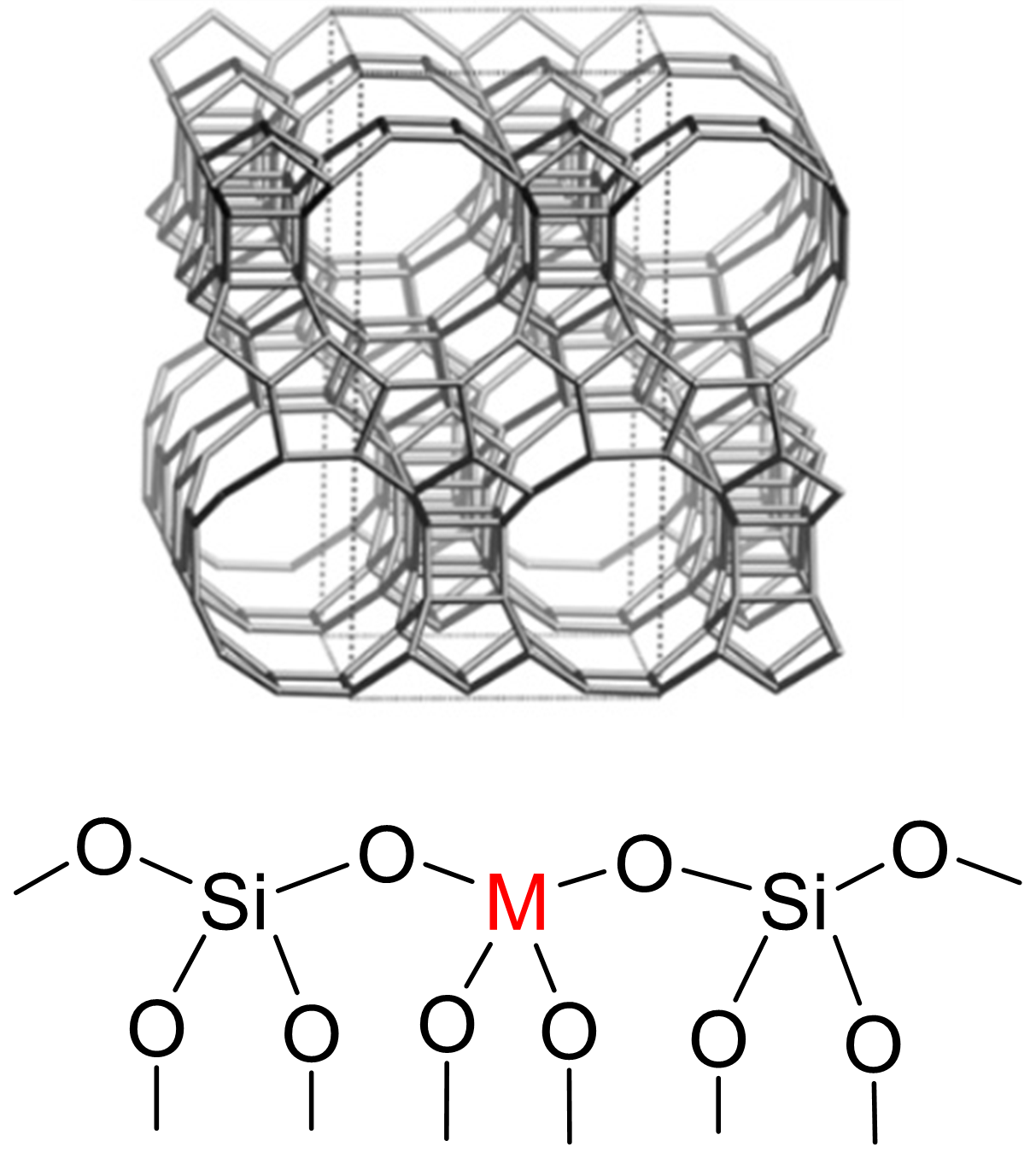
During this thesis project, which was an integral part of the ANR JCJC3 SELECTOSZE project, we sought to determine the mechanisms involved in the formation of these various products of interest, as well as the active species involved. To this end, various M-Beta catalytic materials were synthesized (using different preparation methods and different cations). Kinetic measurements of the conversion of the different sugars (glucose, fructose, xylose) were then obtained, supported by sugar isotope labeling strategies (13C)4 and, in parallel, a molecular modeling study was conducted5.
The first component of the research consisted in comparing the catalytic properties and performance of the various synthesized materials. The optimal preparation method, which allows isolated metal sites to be obtained in the zeolite framework and maximum Lewis acidity to be expressed, depends on the metal in question and its precursor6. Tin (Sn) is the most active of the metals tested, and is best incorporated into the structure using a solid-state preparation method: by grinding the zeolite precursor (a previously dealuminated Beta zeolite) together with an SnCl4 precursor [1]. It is for this reason that the catalyst was selected as the main candidate for further study.
In parallel, molecular modeling work consisted in establishing appropriate models of the active sites, subsequently enabling mechanistic studies to be carried out. To this end, we generated a large number of models of M sites in lattice positions, for M = Sn, Ti, Zr, and Hf, in three distinct crystallographic sites of the zeolite and for various states of hydration, in order to map their domains of existence as a function of temperature and partial water pressure. This made it possible to identify the most appropriate model as a function of the experimental conditions of the catalytic reaction and to determine the main catalytic characteristics (Lewis acidity, in particular) [2-3].
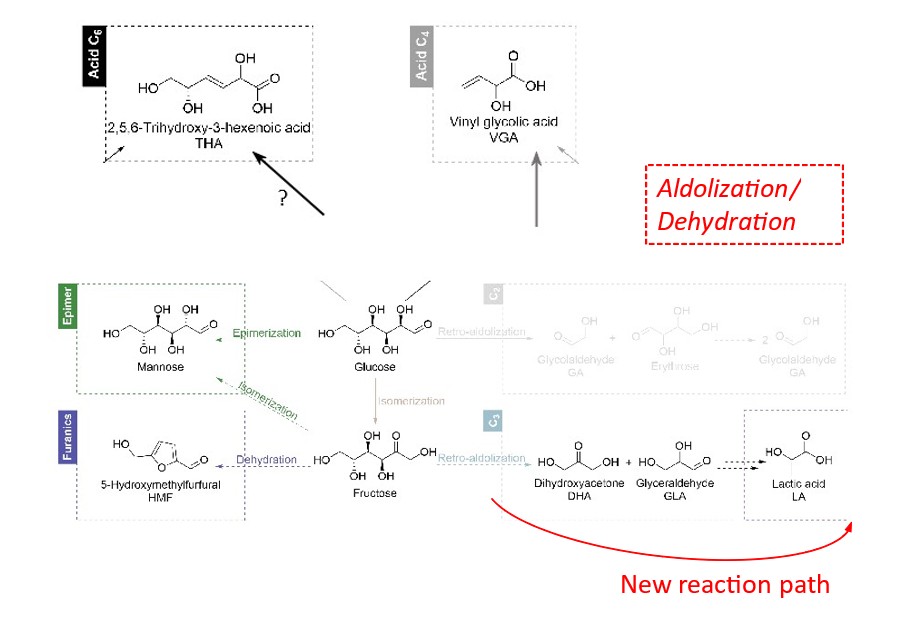
Lastly, the experimental/theoretical mechanistic study was carried out. Careful examination of reaction products obtained using isotopically labeled substrates led to the discovery of new elements not previously reported in the literature and to the proposal of a new reaction process, the main innovations of which are as follows (Figure 2, in red):
- the production of lactic acid involves a kinetically favored key intermediate called enediol rather than the dihydroxyacetone/glyceraldehyde pair often proposed in the literature.
- the products of the glucose retro-aldol reaction (glycolaldehyde and erythrose) are observed in trace amounts in reaction media, not because this reaction does not occur, but because the products evolve into another compound, vinyl glycolic acid (VGA).
It was also possible to demonstrate the chemoselectivity of the retro-aldol reaction, which favors the conversion of ketoses (fructose, xylulose) over that of aldoses (glucose, xylose). We explained this property at the molecular level based on the specific structures of the various sugars and their modes of adsorption at the active sites [4].
This research has improved our knowledge of both the catalytic materials themselves and the main reactions involved in the conversion of biomass sugars. It has already paved the way for further research in the field of sugar conversion, based on the use of bi-functional zeolite materials exhibiting both Lewis and Brønsted acidity 7.
1 A Lewis acid is a chemical species that can accept an electron pair and thus create a covalent bond
2 Reactions involving the isomerization of glucose into fructose, or the epimerization reaction, leading to mannose
3 A project involving young researchers in France
4 For monitoring by Nuclear Magnetic Resonance (NMR) spectroscopy
5 In terms of density-functional theory (DFT)
6 The precursor is the chemical species containing the metal used to prepare the catalyst
7 A Brønsted acid is a chemical species capable of donating a proton
References:
- Y. Boudjema, A. Brunel, R. del Cerro, G. Pirngruber, C. Chizallet, K. Larmier, Relationship between Lewis acid sites and carbohydrate reactivity over Sn-β catalysts, Catalysis Science and Technology, 2024, 15, 396-404,
>> DOI : https://doi.org/10.1039/D4CY01147C
- N. Abidi, Y. Boudjema, C. Chizallet, K. Larmier, Investigating Closed and Open Site Stability of Sn-, Ti-, Zr- and Hf-Beta Zeolites: a Comprehensive Periodic-DFT Study, The Journal of Physical Chemistry C, 2024, 128, 8257-8269,
>> DOI : https://doi.org/10.1021/acs.jpcc.4c02371
- N. Abidi, Y. Boudjema, M. Rivallan, C. Chizallet, K. Larmier, Challenging the distinction between "open" and "closed" Sn-sites in β zeolite by deuterated acetonitrile adsorption: experimental and theoretical insights, The Journal of Physical Chemistry C, 2025, 129, 14011-14019,
>> DOI : https://doi.org/10.1021/acs.jpcc.5c03670
- Y. Boudjema, A. Brunel, N. Abidi, G. Pirngruber, C. Chizallet, K. Larmier, Mechanistic insights of carbohydrates transformations over Sn-β, ACS Catalysis, 2025, accepted
Scientific contact: Kim Larmier





