06.01.2017
2 minutes of reading
Zeolites are silicon and aluminum-based oxide materials that are of major interest in the field of heterogeneous catalysis and whose properties can be altered due to the presence of structural defects. The benefit of these aluminosilicates as catalysts stems from the fact that they present pores of molecular dimensions (up to two nanometers in diameter) - known as micropores - and charge compensating cations (a) that, when they are protons, give the zeolite Brønsted acid properties (b).
The dealumination of zeolites (removal of aluminum atoms from the framework) by steaming is a common post-synthesis operation, widely used to adjust the Brønsted acidity of the solid to a moderate level, in order to stabilize it and also to generate mesopores (larger than micropores, with a diameter of up to 50 nm). These mesopores play a presumed role in the transformation of hydrocarbon molecules that are too big to access micropores. This is the case, for example, in the hydrocracking process, the objective of which is to produce high-quality diesel fuels and kerosene from heavy hydrocarbons.
But the dealumination mechanisms are poorly understood at molecular level, making it difficult to control them in a production context [1]. However, through atomic scale calculations, using ab initio quantum calculation, researchers at IFP Energies nouvelles, working in partnership with Humboldt University in Berlin, elucidated the mechanism involved in the early steps of dealumination for several zeolites.
This study explains how the first water molecule enabling the extraction of an aluminum atom must be coordinated with it according to a specific orientation, in anti-position to the Brønsted acid site (two figures shown left, below) [2]. The capacity of the aluminuma site to be extracted thus depends on steric constraints in this approach orientation. Then, the aluminum atom partially dislodged from its crystallographic position becomes an EFAL (Extra-Framework Aluminum), as a result the participation of at least three additional water molecules, and according to mechanisms potentially even more complex than the very first water molecule approach [3]. Moreover, a second parameter controlling extraction is the stabilization of the EFAL in the pore in which it resides (example of the 12MR mordenite channel (c) shown in the figure on the right).
By making it possible to anticipate the location of sites likely to be dealuminated depending on the nature of the zeolite [3], ab initio evaluation of the free mechanism activation energies provides guides for obtaining more efficient catalysts.
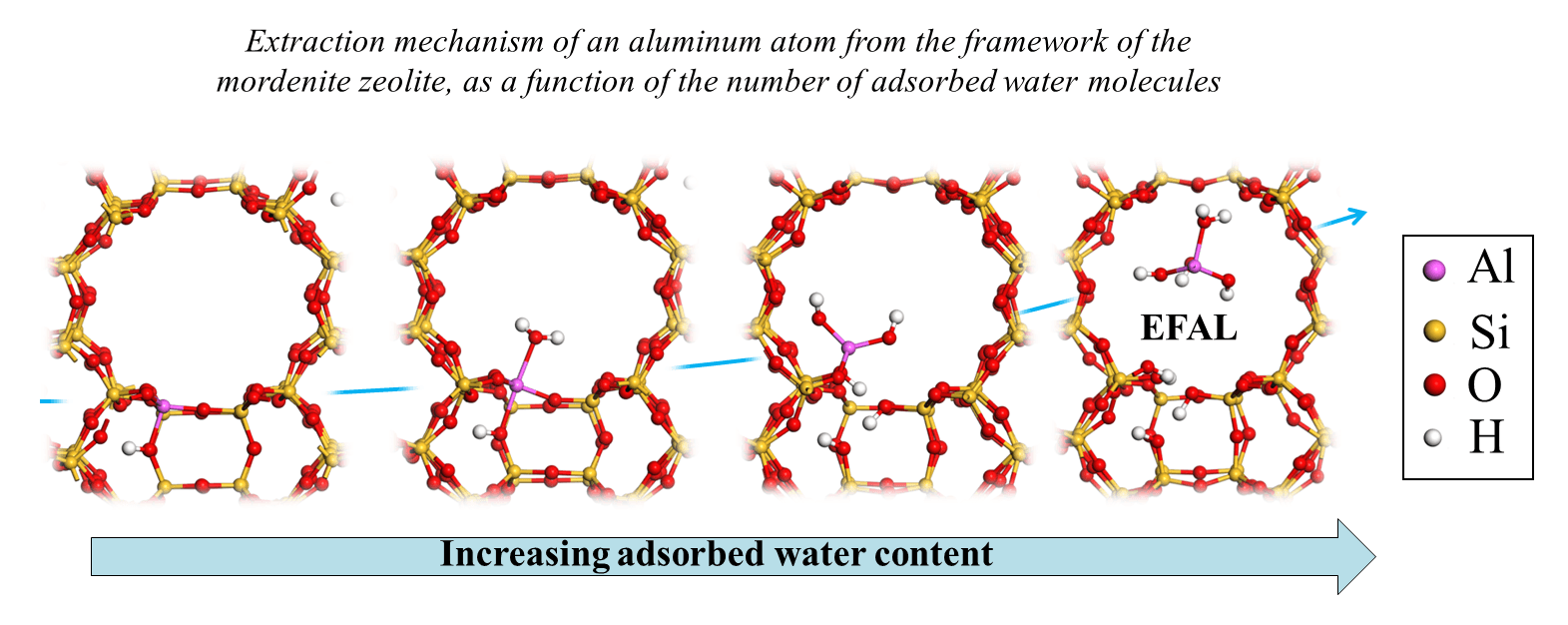
(a) In a zeolite containing aluminum ions, an Al3+ species (formally charged 3 times) replaces an Si4+ ion. Since the solid is an electrically neutral entity, each Al3+ is thus accompanied by a cation charged once positively, for example, to compensate for the charge induced by the substitution.
(B) Capacity to release an H+ proton.
(C) 12MR channel: one-dimensional zeolite pore in the form of a cylinder, delimited by an ellipse comprising 12 silicon and/or aluminum atoms.
(a) Dans une zéolithe comportant des ions aluminium, une espèce Al3+ (formellement chargée 3 fois) remplace un ion Si4+. Le solide étant une entité électriquement neutre, chaque Al3+ est donc accompagné d’un cation chargé une fois positivement par exemple, pour compenser la charge induite par la substitution.
(b) Capacité à céder un proton H+.
(c) Canal 12MR : pore monodimensionnel de la zéolithe en forme de cylindre, délimité par une ellipse comportant 12 atomes de silicium et/ou d’aluminium.
Publications
[1] Challenges on molecular aspects of dealumination and desilication of zeolites
M.C. Silaghi, C. Chizallet, P. Raybaud
Microporous Mesoporous Mater., 191, 82, 2014.
>> http://dx.doi.org/10.1016/j.micromeso.2014.02.040
[2] Regioselectivity of Al-O bond hydrolysis during zeolites dealumination unified
by Brønsted-Evans-Polanyi relationship
M-C. Silaghi, C. Chizallet, E. Petracovschi, T. Kerber, J. Sauer, P. Raybaud
ACS Catalysis, 5, 11-15, 2015.
>> http://dx.doi.org/10.1021/cs501474u
[3] Dealumination mechanisms of zeolites and extra-framework aluminum confinement
M-C. Silaghi, C. Chizallet, J. Sauer, P. Raybaud
J. Catal., 339, 242-255, 2016.
>> http://dx.doi.org/10.1016/j.jcat.2016.04.021





