Thesis by Simon Poirier: « Synthèses en solution d’électrolytes sulfures pour les batteries tout solide » (Wet chemical synthesis of divided sulfides towards All solid state lithium batteries electrolytes)
Solid-state lithium (Li) batteries offer the promise of surpassing the energy density limits of current generations of Li-ion batteries, while being safer. The key to these performances lies in the choice of the solid electrolyte (SE) and its integration into the electrochemical cell. Among the various SEs studied, inorganic thiophosphate phases (Li₃PS₄ and Li₆PS₅X with X = Cl, Br, I)1 are notable for their high ionic conductivity at room temperature (> 10⁻⁴ S.cm⁻¹)1, which makes them suitable for use as solid electrolytes.
Thiophosphates are generally prepared by solid-phase synthesis, combining a milling step and heat treatment. This method produces aggregated micrometric particles. Their integration into hybrid electrolytes (polymer + thiophosphate) or composite cathodes2 therefore requires an additional mechanical milling step to reduce these aggregates and the particle size. To overcome this, one alternative is liquid-phase synthesis, which is known to offer the ability to control both particle size and morphology. However, it appears that this potential has not been fully exploited in the examples reported in the literature for thiophosphate phases.
An in-depth experimental study of the synthesis of liquid Li3PS4/β-Li3PS4 phases revealed key parameters for selecting the appropriate synthesis solvent [1] and better understanding the mechanism of Li3PS4 phase formation in this solvent (tetrahydrofuran or THF) [2]. This synthesis has limitations, the observation and analysis of which led to the development of two innovative synthesis methods.
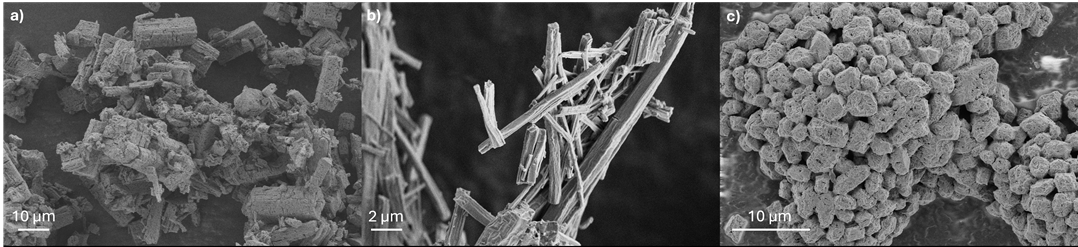
The first method, inspired by hot-injection quantum dot synthesis3, is associated with a 5-fold reduction in the reaction time and, in some cases, eliminates the need for additional heat treatment required to obtain the ionic conductive phase [3]. Morphological control of the particles is achieved through the choice of solvents and/or ultrasound treatment (see Figure 1a and b). This method can be adapted to other phases, such as Li6PS5X argyrodites with X= Cl, Br, I [4].
The second synthesis method is based on solvent exchange. Starting from the intermediate Li3PS4·2THF4, obtained by synthesis in THF, it enables the formation of a wide panel of new “ Li3PS4.xsolvent” intermediates that are not accessible by direct synthesis in the solvents in question. Depending on the choice of solvent, it is possible to control the size, morphology, and ionic conductivity of the final particles (see Figure 1c).
This research has led to advances in the understanding and control of the synthesis of Li3PS4/β-Li3PS4 phases. A new thesis research project will focus on a more downstream aspect: the impact of particle size and morphology on the electrochemical performance of composite cathodes.
1 The Siemens is the unit of measurement for electric conductance in the international system of units. It can be applied to ion conduction
2 A composite cathode consists of an active cathode material (lithium reservoir), a solid electrolyte, and an electronic conductor (carbon)
3 Quantum dots are semiconductor nanoparticles whose color depends on their size. The hot injection preparation method allows this property to be controlled
4 After synthesis of the intermediate Li3PS4·2THF, this intermediate undergoes thermal decomposition, leading either to the amorphous Li3PS4 phase or to the β-Li3PS4phase
References:
- R. Poirier, D. Pasquier, A. Lambert, M. Corral Valero, D. Uzio, C. Garnero, « Solvent Key Parameters for the Wet Chemical Synthesis of the Li3PS4 Solid Electrolyte », J. Phys. Chem. C 2024, 128, 28, 11477–11486
>> DOI : https://doi.org/10.1021/acs.jpcc.4c01598
- R. Poirier, T. Robinson, D. Gajan, A. Lesage, M. Corral Valero, L. Lemaitre, D. Pasquier, A. Lambert, D. Uzio, C. Garnero, « Unveiling Insights in the Formation Mechanism of Li3PS4·2THF Solvato-Complex: H2S Release and Solvent-Phase Interaction », Inorg. Chem. 2025, 64, 7534−7542
>> DOI : https://doi.org/10.1021/acs.inorgchem.5c00445
- R. Poirier, C. Garnero, D. Pasquier, A. Lambert, D. Uzio, FR3157375A1
- R. Poirier, C. Garnero, D. Pasquier, A. Lambert, D. Uzio, FR3157374A1
Scientific contact: Cyril Garnero





