The production of biofuels, renewable diesel or sustainable aviation fuel can be achieved through lipid feedstocks conversion, such as vegetable oils, used cooking oils or animal fats. Through a hydrotreatment stage, we obtain long-chain normal paraffinsa, which must then be isomerised or cracked in order to adjust the properties of the effluent, resulting in the specifications required according to the targeted fuel type (particularly cold flow properties and/or final distillation temperature).
These two reactions are carried out on heterogenous catalysts, which are referred to as “bifunctional” as they incorporate a hydrogenating/dehydrogenating function (noble metal or sulphide of a transition metal) and a Brønstedb acid function (zeolitic and/or amorphous aluminosilicate). The control of their selectivity is therefore essential in order to direct the reaction towards hydro-isomerisation or hydrocracking.
In bifunctional catalysis, selectivity is heavily dependent on the intrinsic properties of the acid phase, i.e. its porosity and Brønsted acidity, but the hydrogenation/dehydrogenation function is also an important factor. Additionally, the catalyst can only achieve its maximum isomerisation selectivity (for given operating conditions) if the hydrogenating/dehydrogenating function is very strong, i.e. of a noble metal-type. This is what we refer to as a well-”balanced” bifunctional catalyst. However, industrial catalysts are often based on transition-metal sulphides, which are more resistant to the impurities present in the feedstock, but less selective. It is therefore essential to be able to counterbalance the impact of this weak hydrogenation function on the activity and selectivity of a bifunctional catalyst, by optimising its formulation or the operating conditions (pressure and temperature).
As part of a collaboration with IST Lisbonc, supplemented by work carried out at IFPEN, a study combining experimentation and modelling examined the interdependence between the hydrogenation function and the zeolitic acid function, in the hydroconversion of n-hexadecane [1]. To this end, catalysts were prepared by the impregnation of zeolites with a strongly hydrogenating element: platinum (Pt). These catalysts were then compared with their low-hydrogenating equivalents, based on molybdenum di-sulphide (MoS2) promoted by nickel.
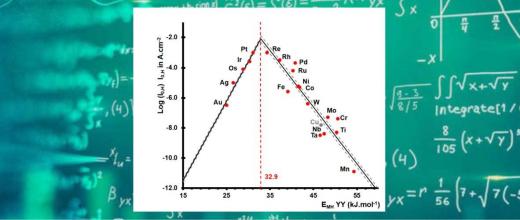
This work resulted in a kinetic model that is capable of predicting the activity and selectivity of the bifunctional catalyst according to the ratio between metallic sites and Brønsted sites (figure 1). This model is also able to predict the behaviour of a bifunctional catalyst involving two different acid phases, which is a highly attractive feature as it offers the ability to fine-tune the selectivity [2] and determine the effects of operating conditions.
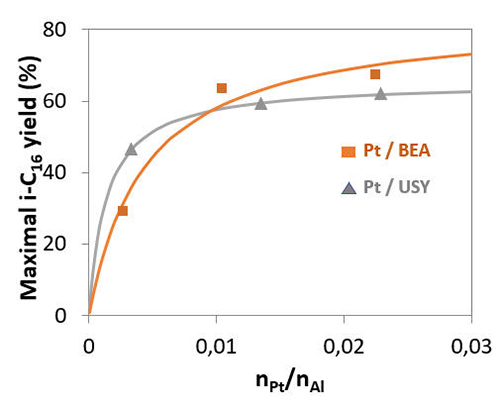
The comparison of Pt catalysts with nickel-promoted MoS2 catalysts [3,4], which are poorly balanced and therefore offer low selectivity towards isomerisation, led to the identification of the yield spread with regard to this reaction and further studies on how it can be remedied. Two combined actions could make it possible to achieve a better balance at process level: the addition of ammonia as an inhibitor of acid sites in the feedstock and the reduction of zeolites in the catalyst support (figure 2). To achieve an identical yield to that of a Platinum-based catalyst, the addition of ammonia to the feedstock reduced the concentration of acid sites by around three orders of magnitude. So, in this example, this is the level that must be achieved in order to balance a nickel-promoted MoS2 catalyst.
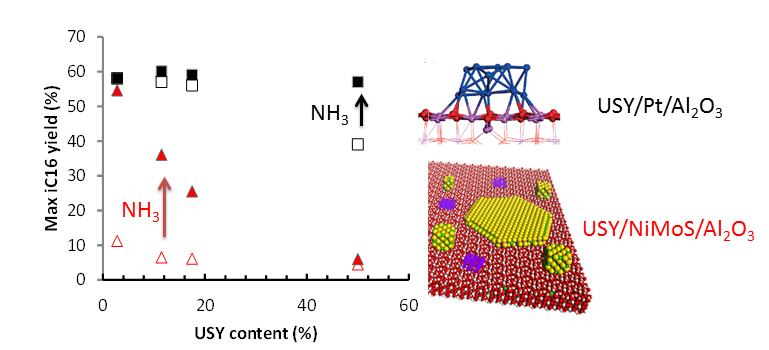
This work has made it possible to quantify the impact of the balance between the hydrogenation function and the acid function of a bifunctional catalyst for the production of biofuels, according to the composition of the catalyst and the operating conditions. Some points that remain partially unclear are still undergoing studies, in particular in relation to the impact of the inhibitors, and the relation between the intrinsic selectivity of the zeolite and the nature of its acid sites.
a- Long-chain linear paraffins (typically more than 10 carbon atoms)
b- “Proton-donor”-type acid
c- Thesis by Pedro Mendes, “Hydroconversion catalysts based on zeolite mixtures, from ideality to reality”, 2017.
References:
- Mendes. P. S. F., Silva. J. M., Filipa Ribeiro. M., Duchêne P., Daudin. A., Bouchy. C., Quantification of metal-acid balance in hydroisomerization catalysts: A step further toward catalyst design. AIChE J. 2017. 63 (7). 2864–2875.
>> DOI: 10.1002/aic.15613
- Mendes. P. S. F., Silva. J. M., Filipa Ribeiro. M., Daudin. A., Bouchy. C., Investigation of cooperative effects between Pt/zeolite hydroisomerization catalysts through kinetic simulations. Catal. Today 2018. 312. 66–72.
>> DOI: 10.1016/j.cattod.2018.02.011
- Mendes. P. S. F., Silva. J. M., Ribeiro. F. H., Daudin. A., Bouchy. C., Bridging the gap between laboratory and industrial hydrocracking: on catalyst and operating conditions effects. Catal. Sci. Technol. 2020. 10. 5136–5148
- Pirngruber. G. D., Maury. S., Daudin. A., Alspektor. P. Y., Bouchy. C., Guillon. E., Balance between (De)hydrogenation and Acid Sites: Comparison between Sulfide-Based and Pt-Based Bifunctional Hydroc
>> DOI: 10.1021/acs.iecr.0c01680
Scientific contacts: Christophe Bouchy, Gerhard Pirngruber, Antoine Daudin
You may also be interested in
Optimal catalyst: another IFPEN contribution for innovative formulations
In situ characterization of the genesis of the active sites of hydrotreatment catalysts by X-ray Absorption Spectroscopy
Meeting environmental standards governing the sulfur content of oil-based fuels hinges around the optimization of hydrotreatment processes (HDT), involving, in parti