Faced with the current climate challenges, alternative fuels are attracting a growing interest for the mobility of the future. Of the various possible alternatives, hydrocarbons could be synthesised via a well-known process: the Fischer-Tropsch (FT) process, based on Syngas (CO and H2) produced, in particular, by biomass gasification. The FT reaction is a polymerisation of CO and H2 that involves chain initiation, propagation and termination reactions leading to a distribution of long-chain hydrocarbon products that can contain up to 100 carbon atoms.
However, the deactivation of FT catalysts is a major issue that directly impacts the costs of the process. This loss of activity is accompanied by a drop in selectivity in synthesised long-chain hydrocarbons, which diminishes the fraction of products that can be recovered. The mechanisms responsible for the deactivation are now well documented, while those behind deselectivation are still poorly understood.
To identify these mechanisms, a multiple-stage methodology was implemented as part of a doctoral thesisa. Firstly, protocols for accelerated ageing were developed to individually simulate each of the key deactivation phenomena. Then, high-throughput experiments (HTE) implementing these protocols made it possible to characterise their effect on the selectivity of the catalysts. Finally, modelling of the experimental data obtained was used to interpret the phenomena involved at molecular level, via a micro-kinetic model detailing each elementary step of the reaction.
The results show that, among the ageing phenomena observed, the deposition of carbon [1], carburation [2] (figure) and oxydation of the active phase all cause a loss of selectivity in long-chain hydrocarbons, with carbon deposition found to have the most adverse affect. However, carburation stands out from the other phenomena as it causes a simultaneous reduction in selectivity toward olefins.
According to the modelling results, it is the modification of chain propagation and termination reaction rates, due to a change in electronic or steric environment of the active sites, that causes the phenomenon of deselectivation. Finally, a model was proposed that was capable of taking the various mechanisms of deactivation and deselectivation into account, which was then used to process industrial data on Fischer-Tropsch synthesis. The resulting simulations reveal that carbon deposition and carburation provide a quantitative explanation for the loss of selectivity observed in experiments.
The identification, characterisation and modelling of deselectivation phenomena are tools that now make it possible to guide the choice of formulation of catalysts and/or operating conditions of the reaction in order to improve the performance of the Fischer-Tropsch process.
Click on the picture to enlarge
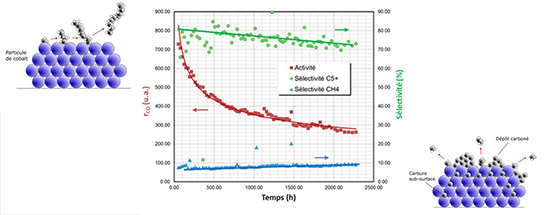
Left: chain growth on new catalyst (t=0)
Right: subsurface carburation and coke deposition perturbating chain growth (end of test)
a- Thesis: “Deselectivation in Fischer-Tropsch catalysis: towards an identification of the mechanisms”, P. Hazemann, Claude Bernard Lyon University 1, 2020.
References:
- P. Hazemann, D. Decottignies, S. Maury, S. Humbert, F. C. Meunier, Y. Schuurman, Journal of Catalysis, 397, 2021, 1-12, ISSN 0021-9517
>> https://doi.org/10.1016/j.jcat.2021.03.005
- P. Hazemann, D. Decottignies, S. Maury, S. Humbert, F. C. Meunier, Y. Schuurman, Journal of Catalysis, 401, 2021, 7-16, ISSN 0021-9517
>> https://doi.org/10.1016/j.jcat.2021.07.009
Scientific contacts: Dominique Decottignies, Sylvie Maury
You may also be interested in
BioTfueL® project: entry into the industrialization and commercialization phase
Characterizing catalysts for Fischer-Tropsch: a question of SWING
Fischer-Tropsch synthesis is a catalytic process to produce hydrocarbons from a syngas, which could come from biomass.
HTE in a milli-fixed bed reactor boosts the development of slurry catalysts
THÈSE DE CHARLES BONNIN






