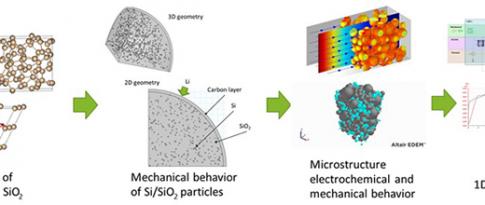
The electrification of mobility is a major transformation aimed at reducing greenhouse gas and pollutant emissions by the transport sector. In this context, the Li-ion battery is currently the technology employed by all car manufacturers to provide the energy storage required for the roll-out of electric vehicles.
However, Li-ion batteries can experience incidents with dramatic consequences, associated with a phenomenon known as thermal runaway. This may be the result of abnormal operating conditions: excessive temperature, mechanical deformation, overcharging, internal short circuit. This thermal runaway is characterized by violent combustion that is difficult to control and produces toxic gas emissions. Today, this is a core safety issue that has a significant impact on battery design and control strategies.
Numerical approaches developed over the past 10 years by IFPEN [1] are used to model and simulate battery behavior during thermal runaway. Nevertheless, the models developed depend on reliable experimental data in order to obtain an understanding and detailed quantitative description of the phenomenon.
It is for this reason that a new thermal runaway characterization system was developed: it operates in an optically accessible chamber and involves high-speed cameras, pressure and temperature sensors and gas analysis systems. The protocol developed makes it possible to subject a cell (unitary component of the battery) to controlled abuse conditions and combine the different types of available measurements.
The results obtained so far have made it possible to identify the four successive phases leading to the thermal runaway phenomenon [2]:
- increase in battery temperature associated with internal degradation reactions;
- voltage decrease in battery terminals;
- ejection of a liquid and gas mixture via the cell’s safety valve (venting);
- actual thermal runaway: strong exothermic reactions leading to the appearance of flames coming out of the battery.
In addition, the high-speed color video enables a qualitative understanding of these different phases and the timescale involved. In particular, the observation of a two-phase jet during venting (phase 3) reveals the role of the gas formed inside the cell: this gas is responsible for the expulsion of a significant part of the liquid/solid phase present in the battery. During the last phase, the images acquired provide indications concerning combustion initiation: it seems to occur outside the defective cell due to incandescent particles expelled by it.


This system and the associated protocol will be used to acquire the data required to produce numerical models that are both predictive and more precise. In particular, PhD research* underway at IFPEN is aimed at developing a quantitative analysis methodology for the gases emitted and obtaining a detailed understanding of the combustion initiation mechanism.
* Thesis title: Development d’un protocole de characterization du phénomène d’emballement IC des Li-ion batteries [Development of a characterization protocol for the thermal runaway phenomenon in Li-ion batteries].
References:
-
Sara Abada, Martin Petit, Amandine Lecocq, Guy Marlair, Valérie Sauvant-Moynot et François Huet, Combined experimental and modeling approaches of the thermal runaway of fresh and aged lithium-ion batteries, Journal of Power Sources, Elsevier, 2018, 399, pp.264-273.
>> https://doi.org/10.1016/j.jpowsour.2018.07.094
-
L. Richardet, M. Bardi, M. Lecompte, V. Brocchetto, S. de Persis, Innovative experimental set up to characterize Lithium-ion batteries thermal runaway, European Combustion Meeting, 2023
Scientific contacts: michele.bardi@ifpen.fr, matthieu.lecomte@ifpen.fr
You may also be interested in
Battery behavior: a complexity best taken into account by modeling
For around fifteen years now, IFPEN has been focusing on modeling conventional batteries to represent their nominal operation (electric and thermal behavior during normal operation), throughout their lifetime (...) and in the event of thermal runaway (failure, improper use)...
SC6 - How to better control loss of lithium battery capacity
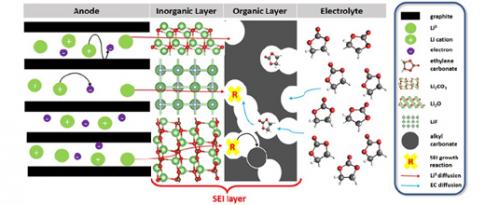
Everybody knows that lithium-ion batteries, used in cell phones, computers, etc., gradually lose capacity and eventually fail. This loss of capacity is primarily due to a layer known as the SEI, which forms between one of the battery’s electrodes and the electrolyte (see Figure). This layer already appears after the first battery charge/discharge cycle, and grows over time, consuming lithium ions. The process is irreversible and therefore detrimental to battery capacitye...